There is nothing more satisfying than the frothy mouth-watering theatre of a beautifully dispensed beer cascading from the tap with all its promise of tasty goodness. Whether a nitro stout, crisp pilsner or a weisse, getting the foam right, with a head that gently follows the beer to the bottom of the glass, is a vital part of the perception and experience of a beer. With competition between brewers fiercer than ever for space on the bar, elements of quality such as foam stability are being put under the microscope by buyers and consumers alike.
There are many aspects relating to beer foam which have long been the subject of study and debate. Here’s the basics you need to know……….
What is foam and how does it form?
Put simply, a foam is a two-phase system in which a gas phase is dispersed in a small amount of liquid in a continuous phase.
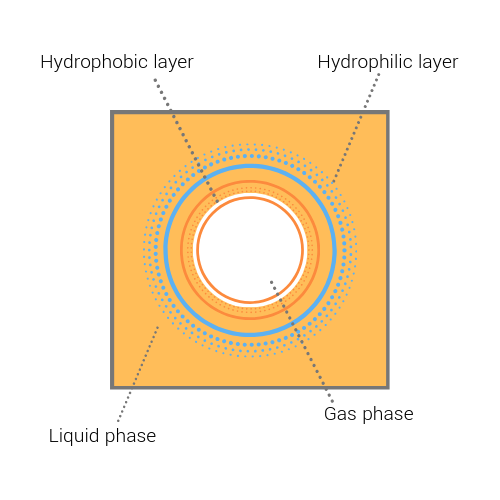
Bubble formation begins when gas in beer becomes insoluble and hydrophobic in beer. For any foam to form, a surface-active foaming agent is crucial – in beer this is protein. Proteins of importance in this case are certain proteins of both High Molecular Weight (HMW) and Low Molecular Weight (LMW), including Lipid Transport Proteins (LTP1, a protein from barley which survives mashing but becomes foam active when de-natured in the boil), hordeins, glutelins, albumins (protein Z).
Some proteins in the beer have a hydrophobic end which is attracted to the gas and a hydrophilic end which is attracted to the liquid surrounding the gas. These proteins align with each other to form the bubble.
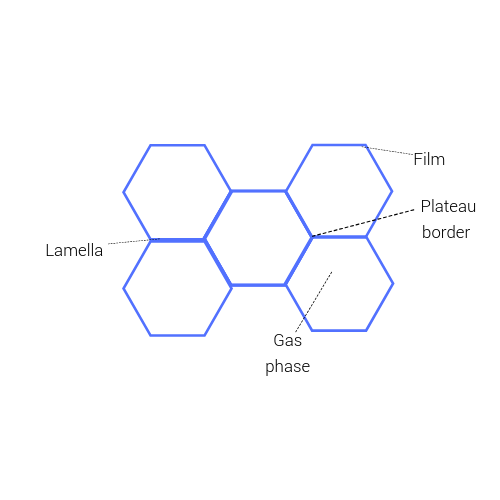
For a stable foam, bubble size has a huge influence on the stability of foam forming a tighter matrix trapping more liquid. Bubble size can be calculated with the following formula.
Bubble Radius = 3x Radius of Nucleation Site x Surface tension 1/3
2x Relative Density of Beer x Acceleration due to gravity
From this you can see that with relatively small variability in the other parameters such as surface tension, by far the biggest influence on bubble size is the size of the nucleation point which give the importance of getting the dispense and glassware correct.
Nitrogen is also greatly important as this will naturally form smaller bubbles, trapping more liquid between bubbles in the foam head making it creamier and more stable. Typical levels of nitrogen in beer of this kind are 15-20ppm.
Creaming
Creaming occurs as foam starts to form at the top of the glass. This is not a single phase process as foam will continue to be replenished by further rising of bubbles as well as foam collapsing. The foam structure is held together by the surface active proteins with the hydrophillic ends starting to stick together, trapping liquid in between them.
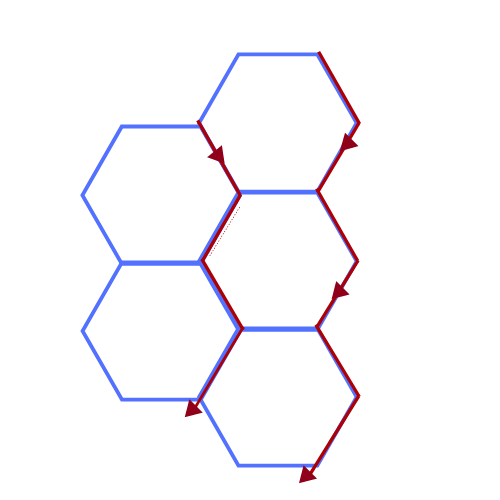
Disproportionation
In any system, gas bubbles have a great propensity to join-together to form larger bubbles due to pressure differentials. This diffusion from small to large bubbles, changes the distribution of the bubbles altering the surface tension leading to liquid drainage.
Drainage
Liquid starts to drain from foam via gravity. This liquid moves along the lamellae to the curves junctions (Plateau borders) where the pressure is lower.
This movement begins the process of the disappearance of the head on the top of the beer.
Importance of Lipids
As there are such a large number of variables in both the chemistry and physics it can be tricky to get the beer head just right. One issue that is not in doubt is the number one enemy of the beer foam are lipids.
If you imagine an oily substance when added to water, it will first form a monolayer on the surface. This is due to its highly amphipathetic (both hydrophobic and hydrophilic) sections with one part of the lipid attracted to the water and the other to air above.
Any small amounts of lipids picked up during the brewing process are likely to be dispersed throughout the beer. The real threat comes from lipids just added to the beer upon or just after dispense. Obvious potential contamination points include dirty glassware (grease and lipstick), inadequately rinsed glassware detergent and oil in dispense gas.
When lipids enter the beer surface and before they start to interact with other molecules on the surface, they will act independently to cause disruption to the surface film causing the surface film to collapse.
If there are sufficient levels of a certain type of proteins called Lipid Binding Proteins (LBP), lipids in the system such as fatty acids and phospholipids will become bound in complexes minimising their foam destabilisation effects. Once these complexes are formed, these lipid-proteins will be carried to the head of the beer via the normal mechanism which will give a stable foam (although not as stable as if there were no lipids present at all).
Helping Foam
- The Grist
It has long been accepted as fact that the use of wheat (such as torrified and malted wheat) in the grist will help improve beer foam (typically up to 10% of grist). This is because wheat is naturally higher in protein and also increases the levels of LBP’s.
A recently published Chinese study (Xiunan Hu, Yuhong Jin, Jinhua Du, Journal of The Institute of Brewing, Dec 2018) examined the foaming character of cloudy wheat beers evaluating the “quality” of foam head for beers of different ratios of wheat and barley malt. They concluded that the foam wasn’t only influenced by the quantity of protein present but also by protein character and origin. They hypothesised that the proteins found in barley malt provide the skeletal structure of the foam matrix with wheat malt protecting and stabilising the foam head. By use of both barley and wheat in the grist, they ensured a good variety of high molecular weight protein and low molecular weight proteins to achieve a good stable foam.
-
Brewing Liquor composition
As always brewing liquor is of great importance to the quality of your product. Having a handle on its composition and its treatment can really make a huge contribution to beer foam quality. Metal ions such as iron will have a very positive effect on the foam, lacing and head retention all enhanced through their ability to bind proteins and iso-alpha acids. However, despite this very positive benefit, these ions can cause no end of further quality issues as well as being potentially toxic. For this reason, metal ions of greatest importance to the brewer are Calcium and Magnesium. These bivalent ions will form the needed ionic bonds in hydrophobic areas encouraging cross linkage between iso alpha acids and proteins. These also have fewer negative impacts on flavour when present in the correct amounts.
The influence of the constituents of brewing liquor on the pH throughout the process should always be given careful consideration in many aspects including beer foam. The pH of the mash dictates protein extraction and enzyme activity and in the boil the pH of the subsequent wort will influence protein coagulation and denaturation.
Nitrogenous material in beer (i.e. protein) will act as natural buffers in low pH, acidic conditions by naturally reacting with hydrogen ions. In foam positive proteins this causes the hydrophobic end to pick up a charge making it hydrophilic. This affects the entire balance of the system that leads to bubble formation so will cause the bubble structure to collapse. This phenomenon explains why some sour beers appear to have poor foaming ability.
-
Get your finings correct
Did you know that isinglass finings have been shown to improve beer foam in poor foaming beers by 15-25%? Not only will this give a superbly bright beer but the addition of isinglass to beer will help bind up and drop out lipid materials for improved foam and flavour stability.
Getting the correct addition rates of kettle finings is also important, over boiling and over dosing of finings can overly reduce content of important proteins in foam formation. As always Murphy’s technical support is here to help get the dose just right to maximise the clarity, ease of processing and foam stability.
-
Use of Murphy’s Antifoam
At first it sounds counter-intuitive that the use of Antifoam will help improve the quality of the foam but each time foam is formed, future foaming potential is lost. Avoiding boil overs or foam loss during fermentation means that important foam positive proteins (often the first to form foams) in processing will be lost from the beer over the side of the vessel.
The formation of foams is prevented, and excessive foam is knocked down with the introduction of highly hydrophobic silica which disrupts and prevents the foam formation.
Use of antifoam also helps keep the brewery clean and can also allow the increase in capacity allowing vessels to be filled to a greater percentage of their overall capacity.
When used at correct levels, it has been proven that particles of antifoam will attach to the yeast cell walls removing them from solution in the beer. This means that the action of the antifoam is completely neutralised and removed with the yeast in normal processing. However, all the beneficial foam positive proteins remain unaffected and in solution in the beer ready to contribute to stable head formation.
-
Hops and Hop Extracts
Owing to their hydrophobicity of iso-alpha acids plays a great part in foam stability. It is thought that these compounds act by bridging the proteins producing addition support to foam structure (this is why the area where the foam meets the beer often tastes most bitter). Reduced hop extracts such as tetra hop can also help improve foam when added post fermentation because these are more hydrophobic so are more effective at supporting the foam structure.
-
Reduce Yeast Autolysis
Yeast autolysis occurs when stressful conditions cause the membranes of vacuoles inside the yeast cell to start to break down. This in turn releases hydrolytic enzymes such as lipases and proteases into the cell which weaken the cell structure causing them to burst open. As well as the potential to add off flavours, this process will also release foam harming proteases into beer.
The strategy to avoid this issue is simple, reduce yeast stress throughout fermentation and reduction of yeast count in finished beer as quickly as possible.
Pitching healthy yeast at the right levels with suitable oxygenation in a temperature-controlled environment free from contaminants to give a consistent fermentation is vital to reduce yeast stress. Another important consideration is use of a good quality yeast nutrient such as Murphy Yeast Aid or Yeast vit. These contain a balanced blend of essential amino acids, zinc and other trace elements and vitamins to ensure that yeast has everything it needs to give a healthy stress-free fermentation.
An often over looked aspect of yeast flocculation is the role of calcium. Multicharged calcium cations promote natural flocculation and dropping of yeast – so once again it’s a case of ensuring there’s enough calcium in the brewing liquor. Effective yeast cropping and use of finings post fermentation will also help reduce break up of yeast cells removing them before this happens.
-
Use of Murphy’s PGA
Propyline Glycol Alginate (PGA) is extracted from the cell walls of brown seaweed and has long been used for foam stabilisation. The action of PGA helps increase head retention and protects against the action of lipids and other foam reducing compounds also greatly improving the lacing of foam down the glass.
PGA is a large polysaccharide molecule which contains carboxyl groups on the glycol alginate which interacts with amino groups on peptides in the bubble wall. The size of this molecule means that its action is much more effective than any influence from hop derived compounds. Like most reactions in the world of bubbles this is electrostatic. Because the polysaccharide is such a large molecule this acts as a bridge cross linking the proteins together helping keep the bubble structures and matrix intact. This also forms a formidable obstacle for lipids to have their disruption influence.
-
In the trade
Assuming the carbonation / nitrogenation levels of beer is correct (after all if there isn’t enough gas in the beer, the bubbles won’t form!) and all other aspects have been addressed in the brewery, the effect of dispense and after sales support should not be underestimated.
Ensuring a lipid free environment from keg to consumer is vital as well as ensuring the correct serving temperature and nucleated glassware will all help ensures beer reaches the consumer in the correct condition.
References and further reading:
O’Rourke (2002), Getting a Head, The Brewer Int Vol.2 (7)
Xuinan Hu, Yuhong Jin, Jinhua Du (2018) Differences in Protein Content….. J. Inst Brewing 550
Bamforth (2012), Perceptions in Beer Foam, J. Inst. Brewing Vol. 110 (4)
Roberts, Keeney, Wainwright (1978), Effects of Lipids & Related Material on Beer Foam J. Inst Brewing Vol. 84 (1)
Jackson, Roberts, Wainwright (1980), Mechanism of Beer Foam Stabilisation By PGA, J. Inst. Brewing Vol 86 (1)
Ang, Bamforth (2014), Foam Inhibitors in Speciality Malts, J. Inst. Brewing Vol 120 (3)
St John Coghlan, Woodrow, Bamforth, Hinchliffe (1992), Polypeptides with Enhanced Foam Potential, J. Inst Brewing Vol 98 (3).
Ballard (1987), Isinglass Types in Relation to Foam Stability, Institute Of Brewing

